Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction H3PO4(aq) + HSO4-(aq) 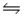
H2PO4-(aq) + H2SO4(aq) .
Ka1(H3PO4) = 7.5 × 10-3; Ka(H2SO4) = very large
A) to the right
B) to the left
C) in the middle
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The oxides SO<sub>2</sub> and N<sub>2</sub>O<sub>5</sub> will form
Q8: A 2.1 L sample of a 0.23
Q21: Which one of the following statements is
Q101: Which of the following solutions is acidic<br>A)
Q108: The salt hydrolysis reaction for CH<sub>3</sub>COONa that
Q111: Calculate the pH of 2.6 × 10<sup>-2</sup>
Q112: Write the chemical formula for the acid
Q120: Which one of these salts will form
Q128: What is the pH of a 0.001
Q145: Which of the following is both a