Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction H3PO4 + NO3-
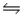
H2PO4- + HNO3 Ka(H3PO4) = 7.5 × 10-3
A) to the right
B) to the left
C) in the middle
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: Calculate the pH of a 0.18 M
Q32: Identify the conjugate acid of SO<sub>4</sub><sup>2-</sup>.<br>A) H<sub>2</sub>SO<sub>4</sub><br>B)
Q37: Of the two acids HBr and H<sub>2</sub>Se,
Q70: Which one of these equations represents
Q93: The pH of tomato juice is about
Q95: Which one of the following equations represents
Q96: Calculate the pH of a 0.021 M
Q98: Given the following K<sub>a</sub> values, which anion
Q116: Which one of these salts will form
Q125: Which one of these net ionic