Multiple Choice
In the reaction CaO(s) + SO2(g) 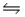 CaSO3(s) ,
CaSO3(s) ,
A) O2- acts as a Lewis base, and SO2 acts as a Lewis acid.
B) Ca2+ acts as a Lewis base, and SO42- acts as a Lewis acid.
C) SO42- acts as a Lewis base, and SO2 acts as a Lewis acid.
D) SO2 acts as a Lewis base, and O2- acts as a Lewis acid.
E) SO2 acts as a Lewis base, and Ca2+ acts as a Lewis acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: The oxides SO<sub>2</sub> and N<sub>2</sub>O<sub>5</sub> will form
Q8: A 2.1 L sample of a 0.23
Q58: Arrange the acids HOBr, HBrO<sub>3</sub>, and HBrO<sub>2</sub>
Q70: Which one of these equations represents
Q96: Calculate the pH of a 0.021 M
Q98: Given the following K<sub>a</sub> values, which anion
Q100: Calculate the pH of a 0.011 M
Q102: Calculate the pH of a 0.20 M
Q116: Which one of these salts will form
Q145: Which of the following is both a