Multiple Choice
The salt hydrolysis reaction for NH4NO3 that represents the acidic or basic nature of the solution is:
A) NH4+(aq) + H2O(l) 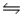 NH3(aq) + H2(g) + OH-(aq)
NH3(aq) + H2(g) + OH-(aq)
B) NO3-(aq) + H2O(l) 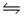 HNO3(aq) + OH-(aq)
HNO3(aq) + OH-(aq)
C) NH4+(aq) + H2O(l) 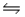 H3O+(aq) + NH3(aq)
H3O+(aq) + NH3(aq)
D) NH4+(aq) + NO3-(aq) 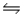 NH4NO3(aq)
NH4NO3(aq)
E) NO3-(aq) + 3H2O(l) 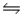 HNO3(aq) + 2H3O+(aq)
HNO3(aq) + 2H3O+(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q3: The pH of a 0.095 M solution
Q47: What is the pH of 10.0 mL
Q56: Due to a highway accident, 150 L
Q58: Which of the following yields an acidic
Q59: What is the pH of a 0.20
Q62: The compound CH<sub>3</sub>NH<sub>2</sub> reacts with water to
Q63: Calculate the pH of a 0.025 M
Q65: Identify the conjugate base of HPO<sub>4</sub><sup>2-</sup>.<br>A) H<sub>2</sub>O<br>B)
Q65: What mass of ammonium chloride must be
Q84: Identify the conjugate base of HSO<sub>4</sub><sup>-</sup>.<br>A) OH<sup>-</sup><br>B)