Multiple Choice
The equilibrium constant for the reaction C6H5COOH(aq) + CH3COO-(aq) 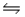
C6H5COO-(aq) + CH3COOH(aq)
Is 3.6 at 25°C.If Ka for CH3COOH is 1.8 × 10-5, what is the acid dissociation constant for C6H5COOH?
A) 5.0 × 10-6
B) 6.5 × 10-5
C) 2.3 × 10-4
D) 8.3 × 10-5
E) 5.6 × 10-6
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Determine the pH of a KOH solution
Q10: Which of the following solutions is acidic?<br>A)[H<sub>3</sub>O<sup>+</sup>]
Q11: The pOH of a solution is 10.40.Calculate
Q12: Calculate the concentration of oxalate ion (C<sub>2</sub>O<sub>4</sub><sup>2-</sup>)in
Q16: Calculate the pH of a carbonated beverage
Q19: What is the H<sup>+</sup> ion concentration in
Q23: The hydronium ion and the hydroxide ion,
Q115: Which of the following is not a
Q137: Hard water deposits (calcium carbonate) have built
Q147: Identify the conjugate acid of CO<sub>3</sub><sup>2-</sup>.<br>A) H<sub>2</sub>CO<sub>3</sub><br>B)