Multiple Choice
Acid dissociation constants for phosphoric acid are given below. 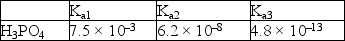 A buffer with a pH = 7.4 can best be made by using
A buffer with a pH = 7.4 can best be made by using
A) H3PO4 and NaH2PO4.
B) NaH2PO4 and Na2HPO4.
C) Na2HPO4 and Na3PO4.
D) only NaH2PO4.
E) only Na2HPO4.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Calculate the molar solubility of AgCl in
Q82: You have 500.0 mL of a buffer
Q83: The solubility of strontium carbonate is 0.0011
Q84: At 25 °C, the base ionization constant
Q84: Calculate the molar solubility of CaF<sub>2</sub> in
Q85: Assuming equal concentrations of conjugate base and
Q87: Will a precipitate form (yes or no)when
Q89: The solubility product for barium sulfate is
Q90: Write a net ionic equation for the
Q91: Write an equation showing the net reaction