Short Answer
Calculate the equilibrium constant Kc for the net reaction shown below.
AgI(s)+ 2NH3(aq) 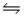
Ag(NH3)2+(aq)+ I-(aq)
For AgI, Ksp = 8.3 × 10-17; for Ag(NH3)2+, Kf = 1.5 × 107.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q49: NaCl is added slowly to a solution
Q50: Calculate the silver ion concentration in a
Q53: Calculate the minimum concentration of Mg<sup>2+</sup> that
Q55: Solid sodium iodide is slowly added to
Q57: For PbCl<sub>2</sub> (K<sub>sp</sub> = 2.4 × 10<sup>-4</sup>),
Q58: The K<sub>sp</sub> for silver(I)phosphate is 1.8 ×
Q59: Calculate the pH at the equivalence point
Q108: The solubility of a salt increases as
Q112: The percent ionization of a weak acid
Q116: Which of the following compounds is more