Multiple Choice
The equilibrium constant at 427°C for the reaction N2(g) + 3H2(g) 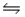 2NH3(g) is Kp = 9.4 × 10-5.Calculate the value of G° for the reaction under these conditions.
2NH3(g) is Kp = 9.4 × 10-5.Calculate the value of G° for the reaction under these conditions.
A) -33 kJ/mol
B) -54 kJ/mol
C) 54 kJ/mol
D) 33 kJ/mol
E) 1.3 J/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Which of the following is consistent
Q101: Which of the following is consistent
Q118: Under which of the following conditions
Q119: Calculate K<sub>p</sub> at 298 K for the
Q120: Nitrosyl chloride (NOCl)decomposes at elevated temperatures
Q122: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img
Q125: <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mi mathvariant="normal">Δ</mi></mrow><annotation
Q126: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q127: Using the thermodynamic data provided below, determine
Q128: Which one of the following reactions