Multiple Choice
Find the temperature at which the reaction N2O4(g) 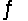 2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm.
2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm. 
A) 300°C
B) 28°C
C) 55°C
D) 32°C
E) 562°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of these species has the highest
Q2: The heat of vaporization of water
Q4: Arrange these compounds in order of increasing
Q5: Which of the following processes would
Q6: Determine the equilibrium constant K<sub>p</sub> at
Q7: Which of these species would you expect
Q8: The element oxygen was prepared by
Q10: For a certain reaction, <span
Q11: Assuming <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q29: Which of the following is consistent