Short Answer
For the reaction CuS(s)+ H2(g) 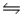
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Assuming <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q12: Ozone (O<sub>3</sub>)in the atmosphere can reaction
Q13: Aluminum forms a layer of aluminum
Q14: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q15: Is the reaction SiO<sub>2</sub>(s)+ Pb(s) <span
Q17: For the reaction 2C(graphite)+ H<sub>2</sub>(g) <span
Q18: Determine the equilibrium constant (K<sub>p</sub>)at 25°C for
Q19: Using the thermodynamic data provided below, calculate
Q20: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q21: The solubility product constant at 25°C