Short Answer
For the reaction SbCl5(g) 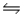
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q51: Using the thermodynamic data provided below, calculate
Q52: Choose the substance with the higher entropy
Q53: The entropy of a perfectly ordered crystalline
Q54: Choose the substance with the higher entropy
Q57: Find the temperature at which K<sub>p</sub>
Q58: Choose the substance with the higher entropy
Q59: Arrange the following substances in the
Q60: Which of the following processes would
Q61: Rubidium has a heat of vaporization
Q122: Which response includes all of the