Multiple Choice
Consider the following standard reduction potentials in acid solution: 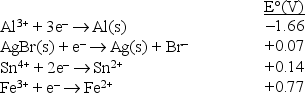 The strongest reducing agent among those shown above is
The strongest reducing agent among those shown above is
A) Fe3+.
B) Fe2+.
C) Br-.
D) Al3+.
E) Al.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: Given the following cell diagram, Al(s)
Q62: For the electrochemical cell Ni(s) | Ni<sup>2+</sup>(1
Q86: Determine the equilibrium constant for the following
Q90: In an electrolytic cell, electrical energy is
Q94: For the reaction Ni<sup>2+</sup>(aq)+ 2Fe<sup>2+</sup>(aq) <span
Q109: Complete and balance the following redox
Q113: Which of the following would be
Q138: Which one of the following reactions
Q143: Complete and balance the following redox
Q145: Complete and balance the following redox