Multiple Choice
Consider the following standard reduction potentials in acid solution: 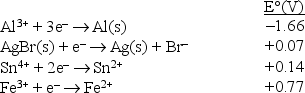 The strongest oxidizing agent among those shown above is
The strongest oxidizing agent among those shown above is
A) Fe3+.
B) Fe2+.
C) Br-.
D) Al3+.
E) Al.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: Consider an electrochemical cell constructed from the
Q119: Calculate the cell emf for the
Q121: Which one of the following reagents is
Q122: Consider a voltaic cell based on
Q125: Complete and balance the following redox
Q127: What is the total number of
Q128: For the electrochemical cell, Fe(s)| Fe<sup>2+</sup>(aq)|| Cu<sup>2+</sup>(aq)|
Q129: Calculate E°<sub>cell</sub> for the following electrochemical cell:<br>Mn(s)|
Q134: Which of the following would be
Q152: Consider the reaction Pb(s) + 2H<sup>+</sup>(aq)