Multiple Choice
Consider the following standard reduction potentials in acid solution: 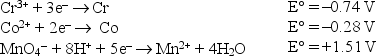 The weakest reducing agent listed above is
The weakest reducing agent listed above is
A) Cr3+.
B) Cr.
C) Mn2+.
D) Co.
E) MnO4-.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Determine the cell diagram for the
Q94: For the reaction Ni<sup>2+</sup>(aq)+ 2Fe<sup>2+</sup>(aq) <span
Q96: Write the balanced overall redox equation for
Q98: Many different ways have been proposed to
Q99: A battery is constructed by placing copper
Q102: A metal object is to be gold-plated
Q113: Which of the following would be
Q139: The half-reaction that should occur at
Q142: Calculate the cell voltage for the
Q145: Complete and balance the following redox