Multiple Choice
Given the following standard reduction potentials, 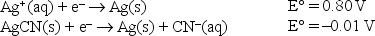 calculate the solubility product of AgCN at 25°C.
calculate the solubility product of AgCN at 25°C.
A) 4.3 × 10-14
B) 2.3 × 1013
C) 2.1 × 10-14
D) 5.1 × 1013
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Complete and balance the following redox
Q17: Determine the cell diagram for the
Q77: How many coulombs (C) of electrical charge
Q97: Consider the following electrochemical cell: U |
Q99: A battery is constructed by placing copper
Q102: A metal object is to be gold-plated
Q107: How many moles of silver metal are
Q108: Calculate E°<sub>cell</sub> for the following reaction:
Q109: How many moles of H<sub>2</sub> are produced<sub>
Q139: The half-reaction that should occur at