Multiple Choice
Consider the reaction 2Fe3+(aq) + Fe(s) 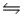 3Fe2+(aq) .Find the equilibrium constant for this reaction at 25°C.
3Fe2+(aq) .Find the equilibrium constant for this reaction at 25°C.
A) 1 × 1011
B) 8 × 1040
C) 3 × 1020
D) 7 × 10-12
E) 1 × 10-41
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: A galvanic cell has the overall
Q49: Complete and balance the following redox
Q86: Predict the products of the electrolysis of
Q122: The measured voltage of a cell
Q129: Calculate E°<sub>cell</sub> for the following electrochemical cell:<br>Mn(s)|
Q131: For the electrochemical cell, Cd(s)| Cd<sup>2+</sup>(aq)|| Co<sup>2+</sup>(aq)|
Q132: Calculate the cell emf for the
Q137: Under standard-state conditions, which of the
Q138: How many minutes would be required to
Q148: Iron objects such as storage tanks and