Essay
Write a balanced equation for a spontaneous reaction which involves the tin and zinc redox couple using the following standard reduction potentials in acid solution 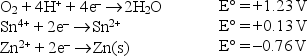
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q100: Which of these metals will not reduce
Q144: How many grams of chromium would be
Q145: Complete and balance the following redox
Q146: Complete and balance the following redox
Q147: Consider the reaction Fe + Sn<sup>2+</sup>(1
Q148: Complete and balance the following redox
Q149: Will H<sub>2</sub>(g)form when Fe is placed in
Q150: Aluminum metal is formed by the electrolysis
Q153: The overall reaction 2Co<sup>3+</sup>(aq)+ 2Cl<sup>-</sup>(aq) <span
Q154: What is the total number of