Short Answer
Determine the equilibrium constant for the following reaction at 25°C.
2I- (aq)+ Br2(l) 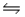
I2(s)+ 2Br-(aq).
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: A galvanic cell has the overall
Q24: What is the total number of
Q42: What is the total number of
Q137: Under standard-state conditions, which of the
Q138: How many minutes would be required to
Q140: How long will it take to produce
Q144: How many grams of chromium would be
Q145: Complete and balance the following redox
Q146: Complete and balance the following redox
Q148: Iron objects such as storage tanks and