Multiple Choice
Assuming a coordination complex is formed with Fe2+ and 1,10-phenanthroline (shown below) , which of the following statements is true? 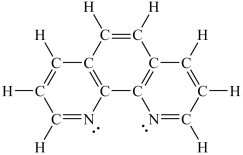
A) If two 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 2.
B) If two 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 6.
C) If three 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 3.
D) If three 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 6.
E) If four 1,10-phenanthroline molecules are coordinated to the iron ion, then the coordination number is 4.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has
Q26: What is the coordination number of chromium
Q29: Predict the number of unpaired electrons in
Q30: The oxidation number of Co in [Co(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]Cl
Q33: What is the oxidation number of Co
Q34: Name the complex ion [CuCl<sub>3</sub>Br(NH<sub>3</sub>)<sub>2</sub>]<sup>2-</sup>.
Q36: Which of these ligands produces the strongest
Q42: The total number of electrons in the
Q88: How many unpaired electrons does the manganese
Q90: In the complex ion [Co(en)<sub>2</sub>Br<sub>2</sub>]<sup>+</sup>, the oxidation