Multiple Choice
The coordination compound shown below has 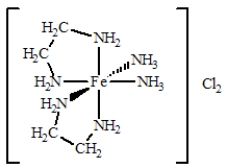
A) two monodentate ligands and two bidentate ligands.
B) three monodentate ligands and two bidentate ligands.
C) four monodentate ligands and two bidentate ligands.
D) one monodentate ligand and four bidentate ligands.
E) four bidentate ligands.
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The neutral monodentate ligand L forms the
Q3: What is the coordination number of silver
Q4: Name the complex ion [Ni(CN)<sub>4</sub>]<sup>2-</sup>.
Q6: What is the oxidation number of Co
Q7: What is the oxidation number of Fe
Q9: What is the oxidation number of cobalt
Q11: Copper can be separated from iron in
Q21: In the coordination compound [Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>], the coordination
Q63: The correct name for [Fe(CO)<sub>6</sub>]Br<sub>3</sub> is:<br>A) hexacarbonyliron(II)
Q72: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has