Multiple Choice
Which of these electron energy level patterns would absorb light with the shortest wavelength? 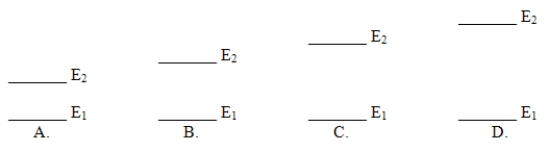
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: The total number of electrons in the
Q17: In the coordination compound [Co(en)<sub>2</sub>Cl<sub>2</sub>]Cl, the coordination
Q35: Which response gives the correct coordination number
Q48: Give the coordination number (C.N.) and oxidation
Q49: How many 3d electrons does a V<sup>3+</sup>
Q55: The total number of electrons in the
Q61: How many 3d electrons does a Mn<sup>2+</sup>
Q80: How would you expect the molecule 1,10-phenanthroline
Q84: What terms describe the geometric isomers that
Q87: Predict the number of unpaired electrons in