Essay
For the complex below determine
(a)the oxidation number of the metal,
(b)the number of d electrons,
(c)the coordination number,
(d)the charge of the complex ion, and
(e)the number and type of ligands for the coordination compound shown below. 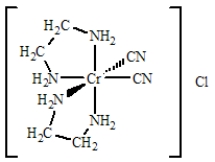
Correct Answer:

Verified
(a)+3,
(b)3 d elect...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
(b)3 d elect...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q11: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has
Q29: In the complex ion [Fe(CN)<sub>6</sub>]<sup>4-</sup>, the oxidation
Q32: Which of these complex ions would absorb
Q33: The correct formula for sodium tetracyanonickelate(II) ion
Q54: The electron configuration of a nickel atom
Q54: Write the chemical formula of the dibromobis(oxalato)cobaltate(III)ion.
Q58: Predict the number of unpaired electrons in
Q62: The electron configuration of an Fe<sup>2+</sup> ion
Q82: The electron configuration of a Ti atom
Q91: A complex with the composition [MA<sub>2</sub>B<sub>2</sub>]X<sub>2</sub> is