Multiple Choice
There are two stable isotopes of chlorine: chlorine-35, with a mass of 34.968853 amu; and chlorine-37, with a mass of 36.965903.Given that the average atomic mass of a chlorine atom is 35.45 amu, which of the following statements is true?
A) Chlorine contains almost exclusively of 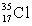 , with very little
, with very little 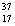 Cl.
Cl.
B) Chlorine contains more 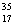 Cl than
Cl than 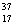 Cl.
Cl.
C) Chlorine contains roughly equal amounts of 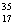 Cl and
Cl and 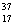 Cl.
Cl.
D) Chlorine contains more 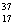 Cl than
Cl than 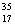 Cl.
Cl.
E) Chlorine contains almost exclusively of 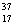 Cl, with very little
Cl, with very little 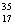 Cl.
Cl.
Correct Answer:

Verified
Correct Answer:
Verified
Q34: In the Haber process, hydrogen gas reacts
Q40: What is the minimum mass of
Q74: How many fluorine atoms are there in
Q158: When a chemical equation is balanced,
Q160: Liquid hexane, C<sub>6</sub>H<sub>14</sub>, burns in oxygen gas
Q162: The mass of 1.21 * 10<sup>20</sup> atoms
Q163: Calculate the molecular mass, in g/mol, of
Q166: Balance the following equation using the
Q167: What is the average mass, in grams,
Q183: Oxidation of a hydrocarbon gave a product