Essay
Nickel has a lower atomic mass than cobalt, even though it has a higher atomic number.One possible explanation is that one of the average atomic masses was miscalculated.In the case of cobalt, there is only one isotope: 100% 59Co at a mass of 58.9332 amu.For nickel, however, there are five isotopes as given in the table. 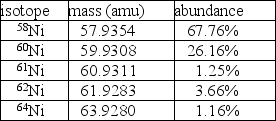 A.Using the data in the table, calculate the average atomic mass for nickel.
A.Using the data in the table, calculate the average atomic mass for nickel.
B.Is the atomic mass for nickel in your periodic table correct?
C.Regardless of your answer to part B, how else could you explain the observation that the atomic mass of nickel is less than the mass of cobalt, even though it has the higher atomic number?
Correct Answer:

Verified
A.58.70 amu
B.yes
C.Cobalt has 27 proton...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
B.yes
C.Cobalt has 27 proton...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q48: Washing soda is a hydrate of sodium
Q49: What is the mass of 0.20 mole
Q52: How many atoms are in 4.39 g
Q53: Balance the following equation using the
Q55: How many grams of sulfur are there
Q56: Balance the following chemical equation:<br>NH<sub>3</sub> +
Q58: Balance the following equation using the
Q63: What is the theoretical yield of
Q72: How many moles of aluminum are present
Q136: Acetylene gas, HCCH(g), can be generated