Short Answer
A chemistry student determined the empirical formula for tungsten oxide (WxOy).To do so, he heated tungsten with oxygen in a crucible.The data that he recorded are shown below: 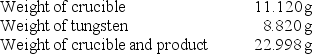 What is the empirical formula of tungsten oxide?
What is the empirical formula of tungsten oxide?
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: The Hall process for the production of
Q38: When balanced with smallest set of
Q39: How many atoms are in 5.54 g
Q41: What is the molar mass of acetaminophen,
Q43: The mass of 1.63 * 10<sup>21</sup> silicon
Q45: Common gases used in laboratories are
Q47: The element oxygen consists of three naturally
Q119: What mass of sodium nitrate would
Q131: How many moles of iron are present
Q176: Phosgene, a poisonous gas used during WWI,