Multiple Choice
Which of the following is the correct net ionic equation for the reaction that occurs when solutions of Pb(NO3) 2 and NH4Cl are mixed?
A) Pb(NO3) 2(aq) + 2NH4Cl(aq) NH4NO3(aq) + PbCl2(s)
B) Pb2+(aq) + 2Cl-(aq) PbCl2(s)
C) Pb2+(aq) + 2NO3- (aq) + 2NH 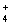 (aq) + 2Cl-(aq) 2NH
(aq) + 2Cl-(aq) 2NH 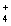 (aq) + 2NO3- (aq) + PbCl2(s)
(aq) + 2NO3- (aq) + PbCl2(s)
D) NH4+(aq) + NO3- (aq) 2NH4NO3(s)
E) No reaction occurs when the solutions are mixed.
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Predict the products of the following
Q35: Define precipitate and illustrate with an example.
Q41: What element is oxidized in the
Q42: Thorium metal is prepared by reacting
Q55: Identify the reducing agent in the
Q56: Lithium metal dissolves in water to yield
Q81: Give an example of a diprotic acid.
Q90: Give an example of a monoprotic acid.
Q118: Give an example of a triprotic acid.
Q147: The oxidation number of Mn in KMnO<sub>4</sub>