Multiple Choice
During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation: 2H2S(g) + 3O2(g) 2SO2(g) + 2H2O(g)
Calculate the standard enthalpy change for the above reaction given: 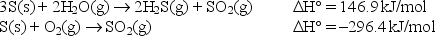
A) -1036.1 kJ/mol
B) -742.3 kJ/mol
C) -149.5 kJ/mol
D) 443.3 kJ/mol
E) 742.3 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Calculate the enthalpy of reaction for
Q25: A beaker contains 115 g of ethanol
Q76: To which one of the following
Q78: An average home in Colorado requires
Q79: The combustion of butane produces heat
Q81: The combustion of butane produces heat
Q82: Calculate the amount of work done, in
Q83: Given that CaO(s)+ H<sub>2</sub>O(l) <span class="ql-formula"
Q84: Given the thermochemical equation 2SO<sub>2</sub> +
Q85: A 0.3423 g sample of pentane, C<sub>5</sub>H<sub>12</sub>,