Multiple Choice
For the reaction 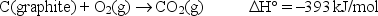 how many grams of C(graphite) must be burned to release 275 kJ of heat?
how many grams of C(graphite) must be burned to release 275 kJ of heat?
A) 22.3 g
B) 0.70 g
C) 12.0 g
D) 17.1 g
E) 8.40 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q14: The heat released when one mole of
Q27: Calculate the amount of heat necessary to
Q28: To which one of the following
Q31: For which of these reactions will
Q31: Calculate the standard enthalpy of formation of
Q32: A 0.3423 g sample of pentane, C<sub>5</sub>H<sub>12</sub>,
Q33: Determine the heat given off to
Q35: Given the thermochemical equation 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)
Q70: Find <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q73: The heat capacity of 20.0 g of