Multiple Choice
The bond enthalpy of the Br-Cl bond is equal to H° for the reaction BrCl(g) Br(g) + Cl(g) .
Use the following data to find the bond enthalpy of the Br-Cl bond. 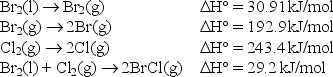
A) 219.0 kJ/mol
B) 203.5 kJ/mol
C) 14.6 kJ/mol
D) 438.0 kJ/mol
E) 407.0 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q14: According to the first law of thermodynamics:<br>A)
Q21: Octane (C<sub>8</sub>H<sub>18</sub>)undergoes combustion according to the
Q22: Find <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q56: A gas is compressed in a cylinder
Q57: If 325 g of water at 4.2°C
Q59: Styrene, C<sub>8</sub>H<sub>8</sub>, is one of the
Q63: How much heat is required to raise
Q64: The combustion of pentane produces heat
Q67: Given H<sub>2</sub>(g)+ (1/2)O<sub>2</sub>(g) <span class="ql-formula" data-value="\rarr"><span
Q113: When an automobile engine starts, the metal