Short Answer
Given the following H° values,
H2(g)+ 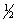 O2(g) H2O(l) H°f = -285.8 kJ/mol
O2(g) H2O(l) H°f = -285.8 kJ/mol
H2O2(l) H2(g)+ O2(g) H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+ 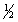 O2(g),
O2(g),
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: Calculate the amount of work done
Q42: Calculate the standard enthalpy change for
Q43: Glycine, C<sub>2</sub>H<sub>5</sub>O<sub>2</sub>N, is important for biological
Q44: The heat of solution of ammonium chloride
Q47: An exothermic reaction causes the surroundings to<br>A)warm
Q48: The total heat of hydration of 1
Q50: Ozone (O<sub>3</sub>)in the atmosphere can be
Q51: Calculate the standard enthalpy change for
Q76: Radiant energy is<br>A) the energy stored within
Q91: A 0.1946 g piece of magnesium metal