Short Answer
Which one of the following sets of quantum numbers is not possible? 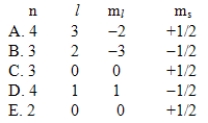
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: The electron configuration of a ground-state vanadium
Q73: Calculate the frequency of visible light having
Q77: The orbital diagram for a ground-state nitrogen
Q78: The Bohr model of the hydrogen atom
Q79: With regard to electron behavior, what happens
Q85: A ground-state atom of manganese has _
Q86: What is the wavelength of radiation that
Q87: What is the wavelength, in meters, of
Q130: Each shell (principal energy level) of quantum
Q131: Which of the following is the electron