Solved
Calculate the Energy Change for the Reaction K(g)+ Br(g) K+(g)+ Br- (G)
Given the Following Ionization Energy (IE)and Electron
Multiple Choice
Calculate the energy change for the reaction K(g) + Br(g) K+(g) + Br- (g)
Given the following ionization energy (IE) and electron affinity (EA) values 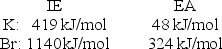
A) -1,092 kJ/mol
B) -95 kJ/mol
C) 95 kJ/mol
D) 1,092 kJ/mol
E) 1,187 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: The number of lone electron pairs in
Q7: The Lewis dot symbol for the calcium
Q13: Nitrous oxide, N<sub>2</sub>O, is sometimes called "laughing
Q35: Which one of the following molecules has
Q37: Which one of the following is most
Q39: Which of the following is a useful
Q42: Assuming the octet rule is obeyed, how
Q53: The electron dot formula for O<sub>2</sub> shows<br>A)
Q69: Which one of the following is most
Q80: Which one of the following ionic solids