Multiple Choice
Use the Born-Haber cycle to calculate the lattice energy of KCl(s) given the following data: H(sublimation) K = 79.2 kJ/mol
I1 (K) = 418.7 kJ/mol
Bond energy (Cl-Cl) = 242.8 kJ/mol
EA (Cl) = 348 kJ/mol
H 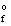 (KCl(s) ) = -435.7 kJ/mol
(KCl(s) ) = -435.7 kJ/mol
A) -165 kJ/mol
B) 288 kJ/mol
C) 629 kJ/mol
D) 707 kJ/mol
E) 828 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: What is the formal charge on the
Q32: Use the Born-Haber cycle to calculate
Q33: What is the formal charge on phosphorus
Q36: Which of the elements listed below is
Q37: A nonpolar covalent bond (i.e., pure covalent)would
Q38: Use the bond enthalpy data given
Q40: Which of the bonds below would have
Q78: The total number of lone pairs in
Q97: Assuming the octet rule is obeyed, how
Q133: Write a Lewis structure for the phosphate