Multiple Choice
Which of the following Lewis structures is incorrect?
A) 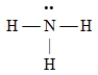
B) 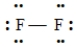
C) 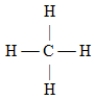
D) 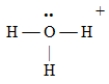
E) 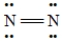
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: The polarity of covalent bonds increases as
Q10: Write a Lewis structure for SO<sub>3</sub> that
Q16: Use bond energies to estimate the enthalpy
Q24: The Si - Cl bond has less
Q28: Write a Lewis structure for the chlorate
Q34: Which of the following solids would have
Q59: Write a Lewis structure for the chlorite
Q94: Which of the following solids would have
Q101: Write a Lewis structure for the phosphate
Q133: How many covalent bonds will be drawn