Short Answer
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. 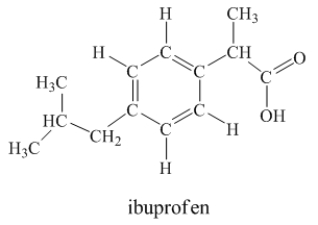 How many sigma bonds and pi bonds are contained in a ibuprofen molecule?
How many sigma bonds and pi bonds are contained in a ibuprofen molecule?
Correct Answer:

Verified
33 sigma b...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q27: Which one of the following molecules is
Q38: The BrF<sub>5</sub> molecule has polar bonds and
Q43: Indicate the number of <span
Q72: Indicate the type of hybrid orbitals used
Q90: Which of the following species has the
Q108: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many
Q110: Predict the molecular geometry and polarity of
Q112: Predict the geometry and polarity of the
Q114: Use VSEPR theory to explain why the
Q115: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many