Multiple Choice
Silver metal crystallizes in a face-centered cubic lattice with L as the length of one edge of the unit cube. The center-to-center distance between nearest silver atoms is
A) L/2
B) 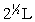
C) 2L
D) 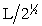
E) None of the above.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q19: The vapor pressure of a liquid in
Q32: Which of the following is not true
Q47: The molar enthalpy of vaporization of hexane
Q48: Of the pair of compounds given, which
Q50: Of the pair of compounds given, which
Q53: Which of the following characteristics indicates the
Q56: The normal boiling point of bromine is
Q57: The normal boiling point of methanol (CH<sub>3</sub>OH)is
Q86: Octane, C<sub>8</sub>H<sub>18</sub>, boils at 125°C as compared
Q118: Indicate all the types of intermolecular forces