Multiple Choice
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 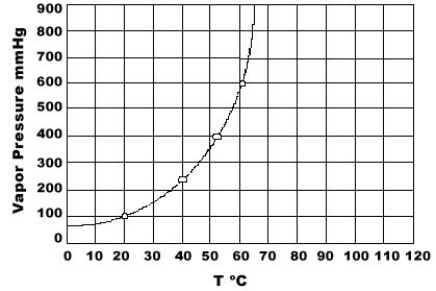
A) 19°C
B) 52°C
C) 60°C
D) 64°C
E) 70°C
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Which liquid is expected to have the
Q41: What phase exists at the point labeled
Q42: Which of the responses includes all of
Q44: Which of the following substances should have
Q46: Suppose the atoms in a two-dimensional crystal
Q47: The molar enthalpy of vaporization of hexane
Q48: Of the pair of compounds given, which
Q50: Of the pair of compounds given, which
Q82: The structural form of the element Ge
Q118: Indicate all the types of intermolecular forces