Short Answer
Based on the phase diagram shown below, which is more dense: the liquid phase or the solid phase? 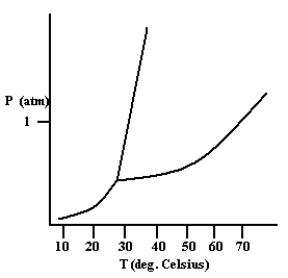
Correct Answer:

Verified
the solid ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
the solid ...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q9: Indicate all the types of intermolecular forces
Q11: Of the pair of compounds given, which
Q16: Of the pair of compounds given, which
Q17: Which of the following substances should have
Q18: The vapor pressure of ethanol is 400
Q29: Identify the dominant (strongest)type of intermolecular force
Q85: Ethanol (C<sub>2</sub>H<sub>5</sub> - OH) will have a
Q113: Indicate all the types of intermolecular forces
Q119: The number of atoms in a body-centered
Q128: Which of the following liquids would have