Multiple Choice
In the reaction H2CO3 + H2O 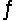 HCO3- + H3O+, the Brønsted acids are
HCO3- + H3O+, the Brønsted acids are
A) H2CO3 and H2O.
B) HCO3- and H2CO3.
C) H2O and H3O+.
D) H3O+ and H2CO3.
E) H2O and HCO3-.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Which of these acids is stronger, H<sub>2</sub>SO<sub>4</sub>
Q22: The OH<sup>-</sup> concentration in a 7.5 *
Q36: Which of these acids is stronger, H<sub>3</sub>AsO<sub>3</sub>
Q52: Consider the weak acid CH<sub>3</sub>COOH (acetic acid).
Q81: Will a 0.1 M solution of NaH<sub>2</sub>PO<sub>4</sub>(aq)be
Q118: The OH<sup>-</sup> concentration in a 1.0 *
Q122: A 0.14 M HNO<sub>2</sub> solution is 5.7%
Q128: A 8.0 M solution of formic acid
Q143: Which solution will have the lowest pH<br>A)
Q172: Calculate the hydrogen ion concentration in a