Multiple Choice
Identify the conjugate acid of CO32- in the reaction CO32- + H2PO4- 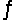 HCO3- + HPO42-
HCO3- + HPO42-
A) H2CO3
B) HCO3-
C) H2O
D) HPO42-
E) H2PO4-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Calculate the concentration of malonate ion (C<sub>3</sub>H<sub>2</sub>O<sub>4</sub><sup>2-</sup>)
Q21: The pOH of a solution is 10.40.Calculate
Q22: If the pH of seawater is 8.0,
Q23: Which of the following yields an acidic
Q24: What is the pH of a solution
Q25: Hydrosulfuric acid is a diprotic acid, for
Q26: Identify the conjugate base of HPO<sub>4</sub><sup>2-</sup> in
Q70: Which one of these equations represents
Q99: What mass of ammonium chloride must be
Q151: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 * 10<sup>-3</sup>,