Multiple Choice
A 0.10 M NH3 solution is 1.3% ionized. Calculate the H+ ion concentration. NH3 + H2O 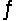 NH4+ + OH-
NH4+ + OH-
A) 1.3 * 10-3 M
B) 7.7 * 10-2 M
C) 7.7 * 10-12 M
D) 0.13 M
E) 0.10 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: Identify the conjugate base of HPO<sub>4</sub><sup>2-</sup> in
Q29: P<sub>4</sub>O<sub>10</sub> is classified as an acidic oxide
Q32: In aqueous solutions at 25°C, the sum
Q33: What is the pH of an aqueous
Q35: Write the chemical formula for nitric acid.
Q54: Which of these acids is stronger, H<sub>3</sub>PO<sub>4</sub>
Q82: Write the formula for the conjugate base
Q92: Calculate the hydrogen ion concentration in a
Q99: What mass of ammonium chloride must be
Q150: Lime is used in farming to reduce