Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction H2SO3(aq) + HCO3- (aq) 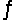 HSO3-(aq) + H2CO3(aq) .
HSO3-(aq) + H2CO3(aq) .
Ka1(H2SO3) = 1 * 10-2; Ka1(H2CO3) = 4.2 * 10-7
A) to the right
B) to the left
C) in the middle
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q24: Which one of these equations represents
Q53: Rain collected on a remote island in
Q96: Write the chemical formula for hydrochloric acid.
Q101: Calculate the pH of a 6.71 *
Q102: Write the chemical formula for sulfuric acid.
Q104: Identify the conjugate base of HSO<sub>4</sub><sup> -</sup>
Q125: Which one of these net ionic
Q125: Arrange the acids HBr, H<sub>2</sub>Se, and H<sub>3</sub>As
Q131: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 * 10<sup>-3</sup>,
Q163: Which one of these salts will form