Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction C6H5COO- + HF 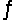 C6H5COOH + F- .
C6H5COOH + F- .
Ka(C6H5COOH) = 6.5 *10-5; Ka(HF) = 7.1 *10-4
A) to the right
B) to the left
C) in the middle
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Due to a highway accident, 150 L
Q6: The oxides SO<sub>2</sub> and N<sub>2</sub>O<sub>5</sub> will form
Q43: Calculate the pH of a 1.6 M
Q59: For maleic acid, HOOCCH=CHCOOH, K<sub>a1</sub> = 1.42
Q63: Morphine, C<sub>17</sub>H<sub>19</sub>NO<sub>3</sub>, is often used to control
Q107: What mass of sodium cyanide must be
Q130: If the pH of stomach acid is
Q155: Predict the direction in which the equilibrium
Q160: In the reaction HSO<sub>4</sub><sup>-</sup>(aq)+ OH<sup>-</sup>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q167: Calculate the pH of a 0.021 M