Multiple Choice
Consider the weak bases below and their Kb values: 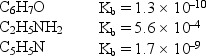 Arrange the conjugate acids of these weak bases in order of increasing acid strength.
Arrange the conjugate acids of these weak bases in order of increasing acid strength.
A) C5H5NH+ < C6H7OH < C2H5NH
B) C6H7OH < C5H5NH+ < C2H5NH
C) C5H5NH+ < C2H5NH3+ < C6H7OH
D) C6H7OH < C2H5NH3+< C5H5NH+
E) C2H5NH3+< C5H5NH+ < C6H7OH
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: If the pH of tomato juice is
Q68: What is the pH of a 0.014
Q79: Arrange the acids HOCl, HClO<sub>3</sub>, and HClO<sub>2</sub>
Q103: Which one of these statements about strong
Q112: Write the chemical formula for the acid
Q136: Which of these lists of molecules is
Q144: What is the pH of a 0.0055
Q144: Which is not a characteristic property of
Q155: Calculate the pH of a 0.14 M
Q157: Which one of these net ionic