Multiple Choice
The equilibrium constant for the reaction C6H5COOH(aq) + CH3COO-(aq) 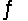 C6H5COO-(aq) + CH3COOH(aq)
C6H5COO-(aq) + CH3COOH(aq)
Is 3.6 at 25°C. If Ka for CH3COOH is 1.8 * 10-5, what is the acid dissociation constant for C6H5COOH?
A) 5.0 * 10-6
B) 6.5 * 10-5
C) 2.3 * 10-4
D) 8.3 * 10-5
E) 5.6 * 10-6
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Which one of the following statements is
Q79: Al(OH)<sub>3</sub> is an amphoteric hydroxide.Write a balanced
Q108: Calculate the pH of a carbonated beverage
Q121: Write the chemical formula for phosphoric acid.
Q122: Acid strength decreases in the series: HNO<sub>3
Q123: An aqueous solution of KCl would be<br>A)neutral<br>B)basic<br>C)acidic
Q128: A solution with a pH of 8
Q129: Which of these acids is the strongest?<br>A)H<sub>2</sub>SO<sub>3</sub><br>B)H<sub>2</sub>SeO<sub>3</sub><br>C)H<sub>2</sub>TeO<sub>3</sub>
Q148: What is the pH of a solution
Q166: Al(OH)<sub>3</sub> is an amphoteric hydroxide.Write a balanced