Essay
Identify the conjugate acid-base pairs in the reaction HSO4- + HF 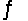 H2SO4 + F-
H2SO4 + F-
One conjugate acid-base pair is _______________; the other acid-base pair is ______________.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q18: What mass of potassium hypochlorite must be
Q36: Which of these acids is stronger, H<sub>3</sub>AsO<sub>3</sub>
Q49: The pH of coffee is approximately 5.0.
Q50: What mass of sodium nitrite must be
Q52: Consider the weak acid CH<sub>3</sub>COOH (acetic acid).
Q62: The compound CH<sub>3</sub>NH<sub>2</sub> reacts with water to
Q67: Which is the formula for the hydronium
Q69: Which response gives the products of hydrolysis
Q129: Calculate the pOH of a solution containing
Q143: Which solution will have the lowest pH<br>A)