Short Answer
For the reaction SbCl5(g) 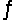 SbCl3(g)+ Cl2(g),
SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 800 K and 1 atm pressure.
Correct Answer:

Verified
1.11  10
10View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: For a certain reaction, <span
Q66: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q67: Hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>)decomposes according to the
Q68: Determine the equilibrium constant (K<sub>p</sub>)at 25°C
Q71: Predict the sign of <span
Q72: Using the thermodynamic data provided below, calculate
Q73: Ozone (O<sub>3</sub>)in the atmosphere can reaction
Q74: Using the thermodynamic data provided below, determine
Q75: K<sub>w</sub> for the auto-ionization of water,
Q98: For the reaction H<sub>2</sub>O<sub>2</sub>(g) <span class="ql-formula"