Multiple Choice
How would you expect the molecule 1,10-phenanthroline (shown below) to function as a ligand? 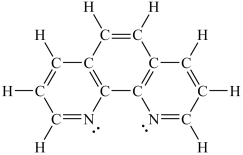
A) It would be expected to be a monodentate ligand.
B) It would be expected to be a bidentate ligand.
C) It would be expected to be a tridentate ligand.
D) It would be expected to be a tetradentate ligand.
E) It would not be expected to function as a ligand.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: What is the oxidation number of cobalt
Q24: In K<sub>4</sub>[Fe(CN)<sub>6</sub>], how many 3d electrons does
Q25: In the following pair of complex ions,
Q31: The systematic name of the coordination compound
Q54: The electron configuration of a nickel atom
Q61: How many 3d electrons does a Mn<sup>2+</sup>
Q71: A molecule or atom that accepts an
Q72: In the complex ion [ML<sub>6</sub>]<sup>n+</sup>, M<sup>n+</sup> has
Q75: Two complex ions containing Ni are
Q88: How many unpaired electrons does the manganese