Multiple Choice
You take 50 mL of small BB's and combine them with 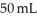 of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
of large BB's and you get a total of 90 mL of BB's of mixed size. Which of the following statements best explains this?
A) Since the density of the small BB's is less than that of the large BB's their volumes do not add directly to one another.
B) This is not possible since the Law of Conservation of Volume would be violated.
C) The total volume actually gets larger since mixing the BB's would leave additional air space because of the difference in size of the two BB sets.
D) Many of the smaller BB's are able to fit within the pockets of space that were empty within the 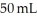 of large BB's.
of large BB's.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Which of the following best describes a
Q7: Red colored Kool-Aid crystals are added to
Q8: Which of the following is something that
Q9: Which of the following does not describe
Q10: A piece of aluminum weighing 10.0 grams
Q12: A supersonic airplane heats up considerably when
Q13: A block of wood that weighs 10.
Q14: What type of phase change does the
Q15: Someone wants to sell you a piece
Q16: Assuming temperature remains constant, what happens to