Multiple Choice
Which has stronger attractions among its submicroscopic particles: a solid at 25°C or a gas at 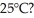
A) The attractions among the submicroscopic particles of the solid must be stronger than the attractions among the particles of the gas.
B) The submicroscopic particles of the gas are moving faster. This means that they have more energy, which means that the attractions among them must be stronger.
C) The attractions among the submicroscopic particles of the solid are much stronger, so much stronger that they hold the particles of the solid absolutely still.
D) The temperatures of these two materials are the same, which means that the attractions among their submicroscopic particles are also of the same strength.
Correct Answer:

Verified
Correct Answer:
Verified
Q17: If all of the above images represents
Q19: Why is it not possible for the
Q20: How does Aristotle's model of matter explain
Q21: Which of the following is an example
Q23: Which of the following is not a
Q24: With increasing temperature the density of air
Q25: Based on experimental evidence, John Dalton postulated
Q26: Dmitri Mendeleev's chart of elements _.<br>A)was used
Q27: Place a card over the open top
Q52: A TV screen looked at from a